This is a war between humans and viruses, and we know that the struggle continues.
Most epidemiologists believe that SARS-CoV-2 will continue to circulate for a long time, and the duration depends on factors such as whether people have immunity to the virus, the duration of immunity, and seasonal changes.
Vaccination is recognized as the most economical and effective way to control the virus epidemic. More than 30 SARS-CoV-2 vaccines are undergoing phase II or phase III clinical trials worldwide. The United Kingdom has approved the use of the new crown vaccine jointly produced by Pfizer and BioNTech and has begun to vaccinate. However, the results of some clinical trials are not optimistic. The neutralizing antibody titer in some subjects is very low, and its protectiveness is still uncertain. At the same time, there is the problem of rapid decline in the neutralizing antibody titer. Therefore, neutralizing the immunized population The detection of antibody titer is of great significance.
Traditional neutralization experiments for evaluating vaccine effectiveness are time-consuming and inefficient. It usually takes 2 to 4 days to complete the evaluation, which brings great inconvenience to large-scale population vaccination effect evaluation. Therefore, there is an urgent need for a simple and rapid alternative method for the evaluation of large-scale population protective antibodies.
Now, Hotgen Biotech can provide you with a product for rapid detection of the new coronavirus SARS-CoV-2 neutralizing antibody, a detection kit based on the colloidal gold method. The kit uses a double antigen sandwich method, and the antigen is a highly specific receptor binding domain of the new coronavirus S protein(S-RBD), which can be used to detect total neutralizing antibodies produced by the human body, including IgA, IgM and IgG. No equipment is required, and accurate test results can be obtained in 15 minutes.
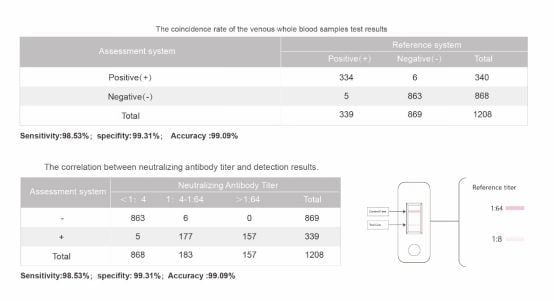
Clinical Performance
Clinical performance of Novel Coronavirus (SARS-CoV-2) Neutralizing Antibodies Test (Colloidal Gold) was determined by testing 339 positive and 869 negative specimens for SARS-CoV-2 neutralizing antibody to have a sensitivity of 98.5%(95%CI: 96.59%~99.52%) and specificity of 99.3%(95%CI:98.50%~99.75%).
At present, the kit has obtained the European Union CE certification, has been launched in the UK and other countries and regions, and has been well received by customers.
At the same time, a kit called SARS-CoV-2 and Flu A/B Antigen Combo Rapid Test (Colloidal Gold) has also been launched on the market. One card can simultaneously detect new coronaviruses and influenza viruses within 15 minutes.
Issued by:
tel: +8618910289851/[email protected]
(Syndicated press content is neither written, edited or endorsed by ED Times)
Read more:
Are Trade Unions Foreseeing Extinction In The Current Work Environment?